New studies reveal how foreign atoms shake-up semiconductors
Michigan State University researchers have revealed new ways that adding trace amounts of foreign atoms, called dopants, can trigger dramatic changes in semiconductors.
These discoveries could reshape the design of materials at the heart of technologies like LEDs, lasers and solar cells, as well as help power future spin-based quantum devices — electronics that use the spin of electrons to store and process information more quickly and with less energy.
“Our new findings challenge the common understanding of the behavior of these emerging materials” said Seokhyoung Kim, an assistant professor in the Michigan State’s Department of Chemistry and senior author of the new studies.
The breakthroughs, published in two companion papers in the journal ACS Nano, explored how dopants transformed the structural, optical and electronic properties of semiconductors belonging to a class of materials known as layered perovskites.
This work was supported by funding from the Oak Ridge Associated Universities and MSU Research Foundation.

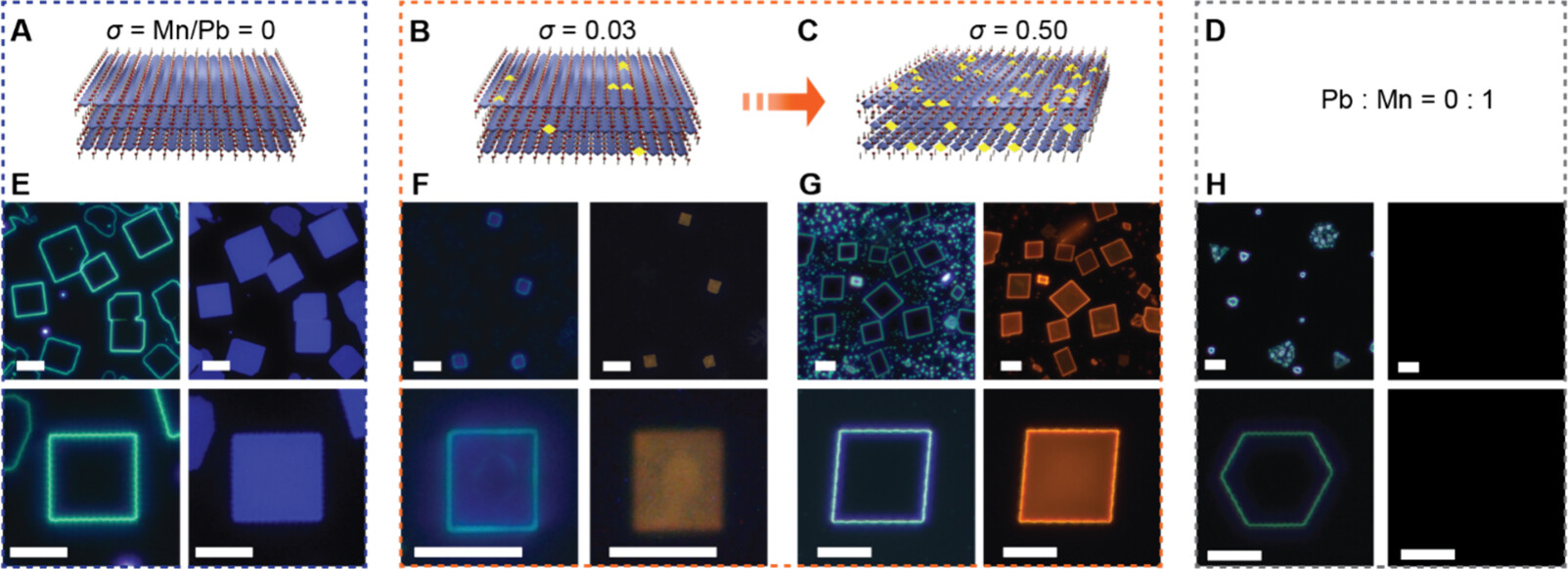
“The normally perfect square shapes of the crystals became slanted, like parallelograms,
and the crystal layers squeezed closer together,” said Pushpender Yadav, a Kim Lab graduate student and first author of the study.
“This amount of change was about ten times larger than what scientists usually see
when doping other semiconductors,” he added.
Using computer calculations, the researchers determined these new crystal structures were more stable than their undoped counterparts, and that this deformation was thermodynamically driven. In other words, the crystals were willingly distorting themselves to find a lower, more stable state of energy.
When tested for light and magnetic properties, the altered crystals also produced a distinct reddish-orange glow as well as a spin alignment along an applied magnetic field — evidence that the manganese atoms were evenly dispersed.
By showing that even a small amount of magnetic atoms could dramatically distort structural and optical qualities, Kim’s team sees exciting potential for controlling semiconductor architecture.
“These insights can guide scientists in designing new materials where structure, magnetism, light emission and magnetism can be precisely tuned,” said Yadav
In a complementary paper, the researchers also explored the properties of the semiconductor cesium bismuth bromide, or CBB.
While a promising, environmentally-friendly alternative to other layered perovskites, in its purest form, CBB is naturally dim and inefficient at emitting light
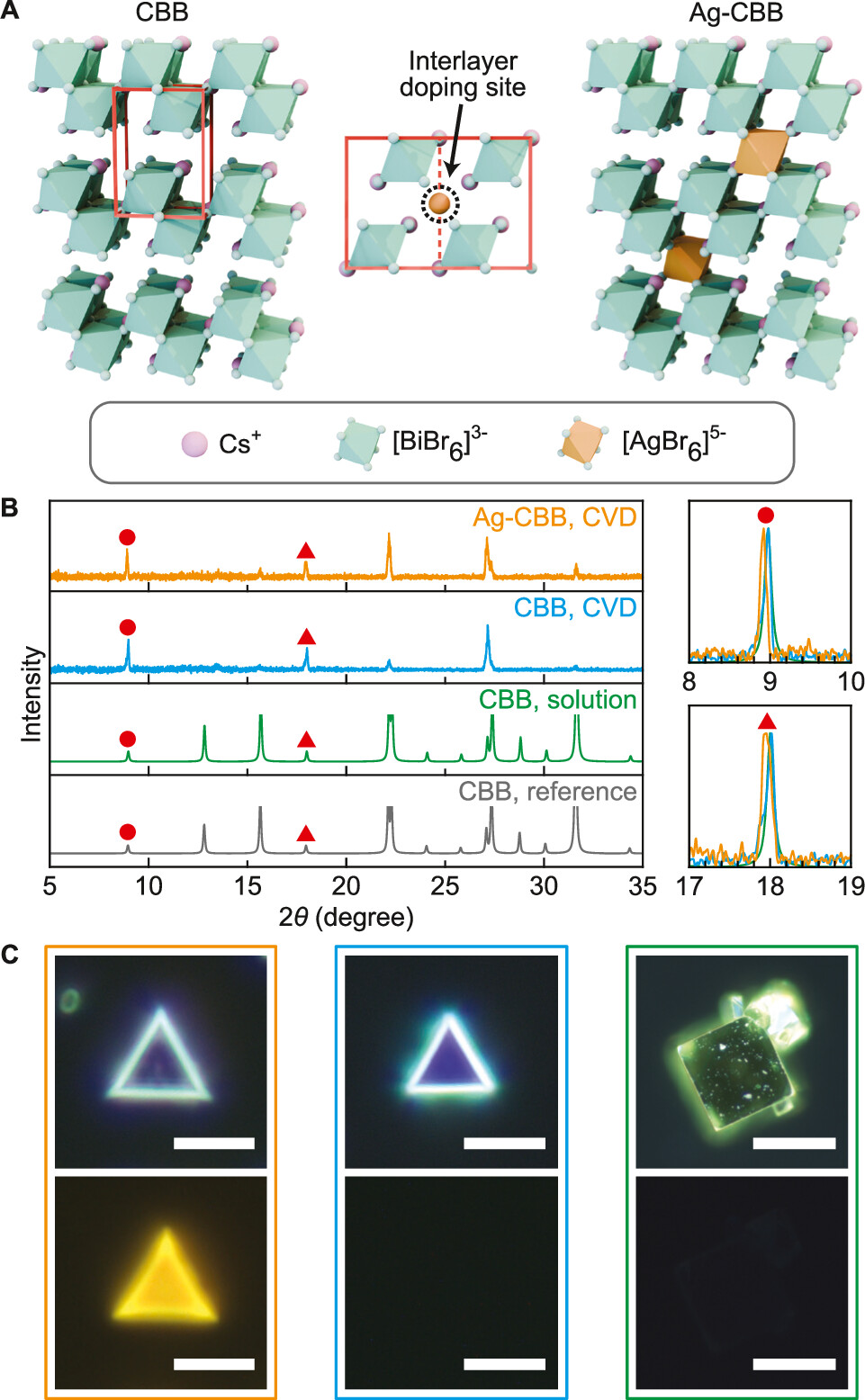
When researchers introduced small amounts of silver dopants to the empty spaces found between layers of CBB, however, the material was dramatically transformed into a bright, broadband light emitter.
Kim’s team discovered that the silver atoms acted a bit like electron traps, holding a unique energy packet of light which the researchers named a bound interlayer exciton, or BIE.
In semiconductors, excitons are quasi-particles made from the coupled pair of an excited electron and the hole it leaves behind. These entities are primarily responsible for absorbing and emitting light.
Compared to the excitons found naturally throughout CBB, the silver-induced BIEs put on a far brighter show.
“We found these excitons emit light more efficiently, and last much longer than the original emission from CBB,” said Kyeongdeuk Moon, first author of the paper and a Kim Lab graduate student.
“By controlling the temperature, we can also tune these excitons and their lifetime,” Moon added, noting that the duration of light emission had increased to an impressive 250 nanoseconds – or over 200 times longer than that shown by native materials.
With an eye toward the future, the Kim Group sees their transformation of CBB as an important step to precisely controlling exciton behavior through doping. Using such techniques, scientists could ultimately create brighter, more efficient and longer-lasting light-emitting technologies ranging from LEDs to lasers.
“These publications have just laid the foundation for the discovery of new functional materials. While there are a series of roadblocks to overcome, the team continues to strive for solutions that will lead to breakthroughs,” said Kim.



